Evidence? Plenty of it.
Testing the El Diablo was no problem, as it zoomed ahead against all of the other battery powered cars that attempted to match it's magnificence. Proof you want? Well this next paragraph will be chalk full of it.
The batteries ability to carry our car to the best capacity outmatched any other battery that we know of so far, proving ours to be the best electric car, an alternative for the ICE (Internal Combustion Engine).
The measuring was done in a hallway outside of the world to prevent public access to our well designed formula. The measurements of blocks was approximately 234 blocks. Each block was a measurement of about .75 meters. Therefor our car less than a foot big was able to travel on one battery charge for about 175.5 meters. How did we get this to work? Read on, reader!
The Battery Charging.
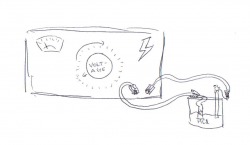
Using a machine to oxidize the the actual Lead Anode, as seen in the diagram, was how we charged the battery before putting it to use.
The Elements of the Car
Lead was always our choice for the superior anode. Lead is also commonly used for batteries as well, such as Lead Acid Batteries. Lead acid batteries are used extensively for cars as well, which makes it an ideal element combined with Oxygen, created PbO2, to create the probably best car.
Aluminum proved to be a better use of a cathode than rather our original plan of Zinc. Aluminium is a soft, lightweight, malleable metal with appearance ranging from silvery to dull gray, depending on the surface roughness. Corrosion resistance is excellent due to a thin surface layer of aluminum oxide that forms when the metal is exposed to air. Not only that! But also Aluminum is supposedly the 3rd most abundant element on the planet so it's resource is quite convenient so in the long run if the El Diablo experiment is mass produced for the public (Theoretically) it would be an easier car battery to make.
